Enhancing spatially-directed evolution of Rubisco in metabolically engineered E. Coli through spatial profile design
Maxwell Furman
Enhancing spatially-directed evolution of Rubisco in metabolically engineered E. Coli through spatial profile design
Maxwell Furman
Spatially-Directed Evolution
Evolution is an incredible mechanism for optimizing the function of organisms, and their composite biomolecular reactions, to survive in novel environments. This process usually happens on time scales that are well past the limits of human observation, however the rapid replication of bacteria serves as a foundation on which evolution can be, and currently is, studied. Over a large spatial gradient, the real-time evolution of E. coli to develop antibiotic resistance has been observed [1]. The nature of bacterial replication allows for the rapid acquisition of mutations, many of which are deleterious or neutral. Alternatively, some may allow for survival in hostile environments, in this case exposure to antibiotics. We aim to utilize this same principle to develop an improved isoform of the enzyme Rubisco.
Rubisco as a Target
Rubisco is the most abundant enzyme on Earth, catalyzing the carbon fixation reaction in photosynthesis. It could be assumed that an enzyme with such a fundamental and long-standing role in the development of life would have been optimized long ago. However, Rubisco has an extremely low rate of enzymatic activity and binds competitively with oxygen, leading to the photorespiration pathway which is less metabolically efficient and actually releases carbon dioxide. Both of these characteristics are potential points of improvements, but for purposes of this project we focus on enhancing the overall catalytic rate of Rubisco which should increase net carbon fixation.
Rubisco-Dependent Metabolic Engineering
The first challenge in pursuing this project was developing a strain of E. coli that was dependent on the function of Rubisco for survival. To accomplish this, we performed a double transformation of cyanobacterial Rubisco and Phosphoribulokinase A (prkA) into DH5 alpha E. coli [2]. The prkA, promoted by the presence of arabinose, diverts the endogenous glycolytic pathway to produce Ribulose bisphosphate (RuBP) which is the substrate for Rubisco and toxic to the cell. Through this logic, higher concentrations of arabinose should promote greater translation of prkA, in turn producing more RuBP which would induce cell death unless Rubisco can mutate to handle the greater enzymatic load. After a successful transformation, we observed this phenomenon under an increasing pressure of arabinose, with a clear minimum inhibitory concentration (MIC) of arabinose appearing. Implementing the stepwise selection gradient described above with arabinose as the selection pressure in place of antibiotics, we hope to direct the evolution of our metabolically engineered E. coli toward a more enzymatically active Rubisco.
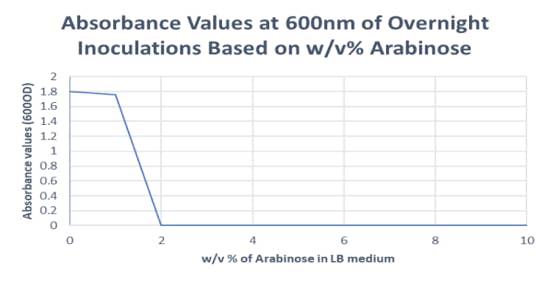
Experimentally determined arabinose MIC. Proof of successful transformation and validation of theory that arabinose can act as a concentration-dependent selection pressure for our metabolically engineered E. coli.
Plate Design
Another goal of this project is to determine how different spatial dimensions and gradient compositions influence the evolution of E. coli, with potential applications in optimizing the effectiveness of spatially-directed evolution experiments. This effect can be most easily analyzed through a gradient of antibiotic concentration, measuring the change in resistance acquisition rate between plate designs. Building on an elementary plate of rectangular segments with logarithmically-scaled stepwise antibiotic concentrations [1], the main design features being explored are varying the width and length of each segment, as well as the boundary length between segments. It is expected that decreasing the width of each segment will increase competition between strains as there is less space and nutrients to share, so only the most dominant strains will prevail. The same can be expected for increasing the segment length, as the more time strains are exposed to each other before being able to access the vacant space, the more dominant strains will take over. However through both these changes, the boundary length must be conserved as greater contact between segments corresponds to larger potential mutational supply. These factors lead to the design of a novel plate, which includes a series of hourglass-shaped segments where the most competitive strains are selected at a bottleneck and allowed to proliferate again until reaching a sufficiently large gradient boundary. Additional features being explored include “Galapagos” isolation mechanisms which allow the bacteria periods of replication without competition to increase mutational diversification, as well as a pattern of hexagonal cells with varying concentrations of antibiotic to offer the bacteria multiple evolutionary pathways.
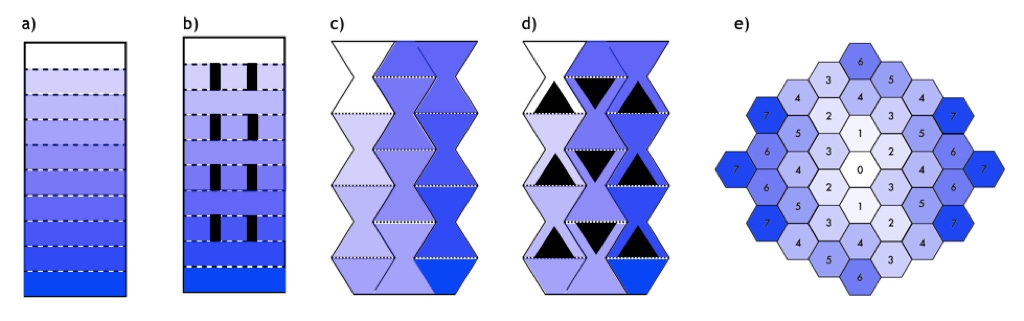
Plate Designs. a) Rectangular Plate with "Galapagos" mechanism. b) Bottleneck Plate. c) Bottleneck Plate with "Galapagos" mechanism. d) Hexagon Plate. e) The dotted lines represent the gradient boundaries, and darker segments represent higher concentrations of antibiotics.
1. Baym, M., Lieberman, T. D., Kelsic, E. D., Chait, R., Gross, R., Yelin, I., & Kishony, R. (2016). Spatiotemporal
microbial evolution on antibiotic landscapes. Science, 353(6304), 1147-1151.
2. Antonovsky, N., Gleizer, S., & Milo, R. (2017). Engineering carbon fixation in E. coli: from heterologous
RuBisCO expression to the Calvin–Benson–Bassham cycle. Current Opinion in Biotechnology, 47, 83-91.
McGill University is located on land which has long served as a site of meeting and exchange amongst Indigenous peoples. We honor, recognize, and respect these nations as the traditional stewards of the lands and waters on which we meet today.
McConnell Engineering Building
3480 University Street, Room 350
Montreal, Quebec H3A 2A7
Phone: 514-714-8239
Fax: 514-398-7379
Email: allen.ehrlicher@mcgill.ca
Office: McConnell Engineering Building 358