Cytoskeleton mechanics via actin dynamic crosslinking
Hossein Khadivi Heris
Cytoskeleton mechanics via actin dynamic crosslinking
Hossein Khadivi Heris
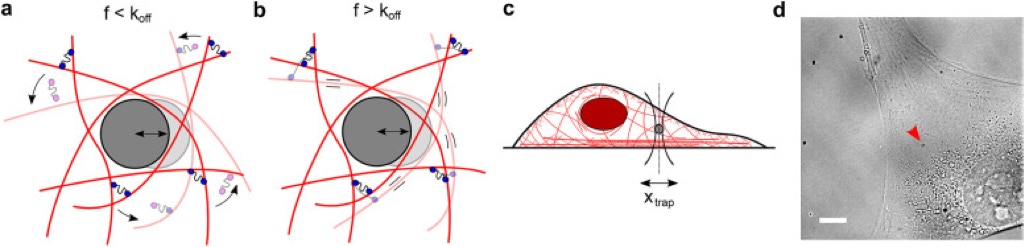
The cytoplasm is a crowded viscoelastic matrix of cross-linked polymers and proteins. (a) Schematic of the actin network being deformed by a microbead at frequencies (f) slower than the unbinding rate of the cross-linkers (koff), before (opaque) and after deformation (transparent). Previously bound cross-linkers (blue heads) detach (magenta heads), and the filaments are free to slide past one another and rearrange (curved arrows), resulting in a more viscous, fluid-like network. (b) At frequencies faster than koff, the cross-linkers stay bound and interlock the filaments, resulting in filament bending and a more solid-like, elastic response. (c) Schematic of the experimental setup showing the active oscillation of bead in the intracellular environment by the OT (xtrap). Not to scale. (d) Representative brightfield image of a live cell showing the optically trapped bead (arrow). Scale bar is 10 µm. [1]
Cells precisely control their mechanical properties to organize and differentiate into tissues. The architecture and connectivity of cytoskeletal filaments change in response to mechanical and biochemical cues, allowing the cell to rapidly tune its mechanics from highly cross-linked, elastic networks to weakly cross-linked viscous networks. While the role of actin cross-linking in controlling actin network mechanics is well-characterized in purified actin networks, its mechanical role in the cytoplasm of living cells remains unknown. Here, we probe the frequency-dependent intracellular viscoelastic properties of living cells using multifrequency excitation and in situ optical trap calibration. At long timescales in the intracellular environment, we observe that the cytoskeleton becomes fluid-like. The mechanics are well-captured by a model in which actin filaments are dynamically connected by a single dominant cross-linker. A disease-causing point mutation (K255E) of the actin cross-linker α-actinin 4 (ACTN4) causes its binding kinetics to be insensitive to tension. Under normal conditions, the viscoelastic properties of wild-type (WT) and K255E+/- cells are similar. However, when tension is reduced through myosin II inhibition, WT cells relax 3× faster to the fluid-like regime while K255E+/- cells are not affected. These results indicate that dynamic actin cross-linking enables the cytoplasm to flow at long timescales. [1]

The binding affinity between wild type (left) and K255E (right) ACTN4 with actin filaments. Principle actin binding domains are shown in blue, with the third putative site in yellow. The K255E mutation removes the bridge (green top image) allowing the third putative actin-binding domain (yellow) to be exposed, which increases the cross-linker affinity for actin. [2]
1. Chaubet L, Chaudhary AR, Heris HK, Ehrlicher AJ, Hendricks AG. Dynamic actin cross-linking governs the
cytoplasm's transition to fluid-like behavior. Mol Biol Cell. 2020 Jul 21;31(16):1744-1752.
2. Ehrlicher AJ, Krishnan R, Guo M, Bidan CM, Weitz DA, Pollak MR. Alpha-actinin binding kinetics modulate
cellular dynamics and force generation. Proc Natl Acad Sci U S A. 2015 May 26;112(21):6619-24.
McGill University is located on land which has long served as a site of meeting and exchange amongst Indigenous peoples. We honor, recognize, and respect these nations as the traditional stewards of the lands and waters on which we meet today.
McConnell Engineering Building
3480 University Street, Room 350
Montreal, Quebec H3A 2A7
Phone: 514-714-8239
Fax: 514-398-7379
Email: allen.ehrlicher@mcgill.ca
Office: McConnell Engineering Building 358